Free Anesthesia Exam Sample Questions
See why TrueLearn is a trusted resource for thousands of medical students and residents. We understand that it’s all about the content. That’s why we have high-yield anesthesia practice questions written and screened by physician authors, which are regularly updated to ensure our SmartBanks stay up-to-date with exam blueprint changes. Below is are free anesthesia exam sample questions to showcase what we mean.
Try This ABA ITE Sample Question from the 2026 New Edition
Which of the following is an absolute contraindication to neuraxial anesthesia?
A. Aortic stenosis
B. Increased intracranial pressure due to intracranial mass
C. Inherited coagulopathy
The Answer and Explanation
Did you get it right? The correct answer is B.
Increased intracranial pressure caused by an intracranial mass is an absolute contraindication to neuraxial anesthesia because brainstem herniation can subsequently ensue.
Proper patient selection for neuraxial anesthesia is of the utmost importance. Absolute contraindications to neuraxial anesthesia include increased intracranial pressure (except in cases of idiopathic intracranial pressure), patient refusal, infection at the site of injection, uncorrected hypovolemia, and allergy to any drugs involved. Relative contraindications should be weighed against the potential benefits and include coagulopathy, sepsis, fixed cardiac output states, aortic stenosis, and neurological diseases. Preexisting neurologic conditions may also be considered a relative contraindication to neuraxial anesthesia, but the individual patient’s disease severity needs to be considered.
| Contraindications to Spinal Anesthesia | |
| Absolute Contraindications | Relative Contraindications |
| – Patient refusal – Infection at the site of injection – Uncorrected hypovolemia – Allergy to drugs used – Increased intracranial pressure* | – Coagulopathy – Sepsis – Fixed cardiac output states – Aortic stenosis – Indeterminate neurological disease** |
| * Except in cases of idiopathic intracranial pressure. ** Such as multiple sclerosis and other demyelinating diseases. | |
Incorrect Answer Explanations
Answer A: Aortic stenosis is a relative contraindication to neuraxial anesthesia. Since patients with aortic stenosis are preload dependent, the speed and extent to which systemic vascular resistance can be reduced in spinal anesthesia can lead to a rapid decrease in coronary perfusion. Thus, each case should be assessed individually based on the severity of the disease. An epidural or intrathecal catheter can be considered, and small doses can be titrated over a longer time to avoid drastic hemodynamic changes.
Answer C: Inherited coagulopathy, such as hemophilia, idiopathic thrombocytopenic purpura, or von Willebrand disease, is a relative contraindication to neuraxial anesthesia. There are no minimum safe levels for these factors or platelet counts. However, hemorrhagic complications are infrequent if the levels are > 0.5 international units/mL for factor VIII or von Willebrand factor or when the platelet count is > 50,000/μL.
Bottom Line
Absolute contraindications to neuraxial anesthesia include patient refusal, infection at the injection site, uncorrected hypovolemia, allergy to any drugs involved, or increased intracranial pressure (except in cases of idiopathic intracranial pressure).
Experience TrueLearn’s 2025 ABA ITE SmartBank Edition today! Save 10% off with code ABA2025.
Subscribe & save today!Free Anesthesia BASIC Sample Question
Which of the following changes will cause the GREATEST decrease in cerebral blood flow during otherwise normal physiologic conditions?
- A) Decreasing PaO2 from 80 to 45 mm Hg
- B) Decreasing mean arterial blood pressure from 100 to 70 mm Hg
- C) Decreasing body temperature from 37 to 34 degrees Celsius
- D) Increasing PaCO2 from 40 to 60 mm Hg
The Answer and Explanation
The correct answer is: C
Decreasing body temperature from 37 to 34 °C will lead to the most significant decrease in cerebral blood flow (CBF).
Cerebral blood flow, under normal circumstances, is tightly coupled to cerebral metabolism, typically represented by the cerebral metabolic rate of oxygen (CMRO2). Many factors that affect cerebral metabolism, therefore, affect CBF. Notably, CMRO2 and CBF are affected by temperature such that for every one °C decrease in temperature, CMRO2 decreases by approximately 6% with a proportional decrease in CBF. This has led to extensive use of hypothermia for multiple types of surgeries including cardiac, thoracic aneurysm repair, and cerebral aneurysm repair.
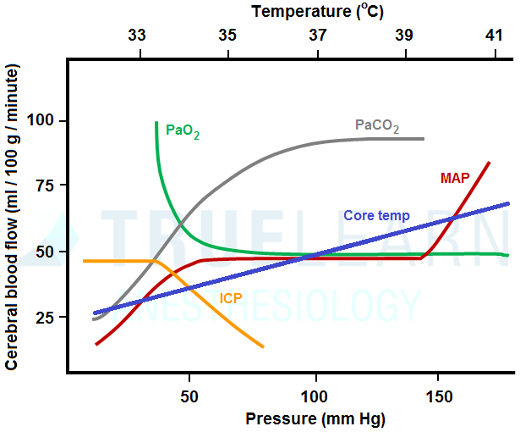
Cerebral blood flow is also directly affected by a number of other factors that do not affect metabolism including arterial partial pressures of carbon dioxide (PaCO2) and oxygen (PaO2) and mean arterial blood pressure (MAP).
Within a normal physiologic range, changes in CBF are directly proportional to changes in PaCO2: for every 1 mm Hg change in PaCO2, CBF changes by approximately 3%. Accordingly, increasing PaCO2 from 40 to 60 mm Hg will cause an increase in CBF of approximately 60%. This effect is relatively short-lived, however, as CSF pH will normalize over a period of minutes to hours and CBF will subsequently return to baseline.
Compared to PaCO2, PaO2 has much less of an effect on CBF. Cerebral blood flow is essentially constant with any PaO2 >50-60 mm Hg. However, CBF rises dramatically when PaO2 decreases below 50 mm Hg.
Cerebral blood flow is maintained relatively constant over a wide range of arterial blood pressures under normal circumstances. This effect is called autoregulation and functions to maintain stable CBF. Under normal circumstances, the range of MAPs over which autoregulation occurs and at which CBF is kept constant is approximately 50-150 mm Hg, though this varies by individual. Therefore, decreasing MAP from 100 to 70 mm Hg will have no effect on CBF. Outside of the autoregulatory range, CBF becomes directly dependent on arterial blood pressure.
- Answer A: CBF rises dramatically when PaO2 decreases below 50 mm Hg.
- Answer B: This will have no effect on CBF.
- Answer D: This will increase CBF.
Bottom Line
Cerebral blood flow is directly related to body temperature, PaCO2 (within normal physiologic ranges), and extremes of MAPs (< 50 or >150 mm Hg). Cerebral blood flow is inversely related to PaO2 when less than 50 mm Hg. Cerebral blood flow remains unchanged within the autoregulatory range of MAPs (50-150 mm Hg) and with PaO2 >50 mm Hg.
For more information, see:
American Board of Anesthesiology Keyword. “Cerebral Blood Flow: Temp Effect” Barash, Clinical Anesthesia, 6th edition, pp. 1006-1008, 1014-1015.
Free Anesthesia ADVANCED Exam Sample Question
A 31-year-old male with type I diabetes is admitted to the ICU after surgery for Fournier gangrene. Following 30 mL/kg of crystalloid administration, the patient’s vital signs are:
- Heart rate: 90
- Blood pressure: 80/45 mmHg
- Cardiac index: 3.0 L/min/m2
- CVP: 8
- SaO2: 98%
- SvO2: 78%
Which of the following is the BEST strategy for providing vasopressor support?
- A. Epinephrine first then add vasopressin if MAP remains below goal
- B. Norepinephrine first then add vasopressin if MAP remains below goal
- C. Vasopressin first then add epinephrine if MAP remains below goal
- D. Vasopressin first then add norepinephrine if MAP remains below goal
The Answer and Explanation
The correct answer is: B
According to the 2016 Surviving Sepsis Campaign guidelines, norepinephrine is recommended as the first line vasopressor to achieve a MAP of ≥65 mm Hg. If adequate MAP is not achieved, vasopressin or epinephrine is recommended as a second-line pressor.
The 2016 Surviving Sepsis Campaign emphasized that sepsis and septic shock are medical emergencies for which resuscitation and treatment should be begun immediately. The recommended initial management for sepsis-related hypoperfusion is the administration of at least 30 mL/kg of IV crystalloid within the first three hours. Following this initial resuscitation, additional fluid administration should be guided by the frequent reassessment of the patient’s hemodynamic status.
For patients with septic shock, vasopressor therapy should be started to initially target a MAP ≥ 65 mm Hg. Norepinephrine is recommended as the first-choice vasopressor since it is associated with reduced mortality and is less likely to cause arrhythmias or splanchnic ischemia than other pressors including dopamine, epinephrine, and vasopressin. If norepinephrine infusion does not raise MAP to target, the guidelines suggest adding either vasopressin or epinephrine.
Answers A & C & D: Epinephrine and vasopressin are not recommended as the first-line vasopressor for patients with septic shock.
Bottom Line
The 2016 Surviving Sepsis Campaign recommends norepinephrine as the first-line vasopressor for patients with septic shock after initial fluid resuscitation. If norepinephrine administration does not raise the MAP to the initial target of ≥65 mm Hg, the guidelines suggest adding either vasopressin or epinephrine.
TrueLearn Insight
The Surviving Sepsis guidelines have gone through several revisions with changes to the second-line vasopressor agent. In the first guidelines, vasopressin was suggested as the second line agent. In the second definitions, the vasopressin dose was decreased and epinephrine was suggested as the second line agent. In the third definitions, either vasopressin and epinephrine are suggested as second-line options. Throughout all the guideline changes, norepinephrine has remained the initial agent of choice for septic shock.
For more information, see:
American Board of Anesthesiology Keyword. “Septic Shock: Vasopressin rx”
Rhodes A, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017 Mar;45(3):486-552.